Streptavidin
- Home
- Streptavidin
Streptavidin
Streptavidin is a biotin-binding protein found in culture broth of the bacterium Streptomyces avidin. Streptavidin binds 4 moles of biotin per mole of protein with a remarkably high affinity. Streptavidin has isoelectric point near to neutrality where most useful biological interaction occur. Accordingly, streptavidin exhibits lower levels of non-specific interaction than does avidin molecule when the streptavidin protein used in applications relying upon the formation of avidin/biotin complexes.
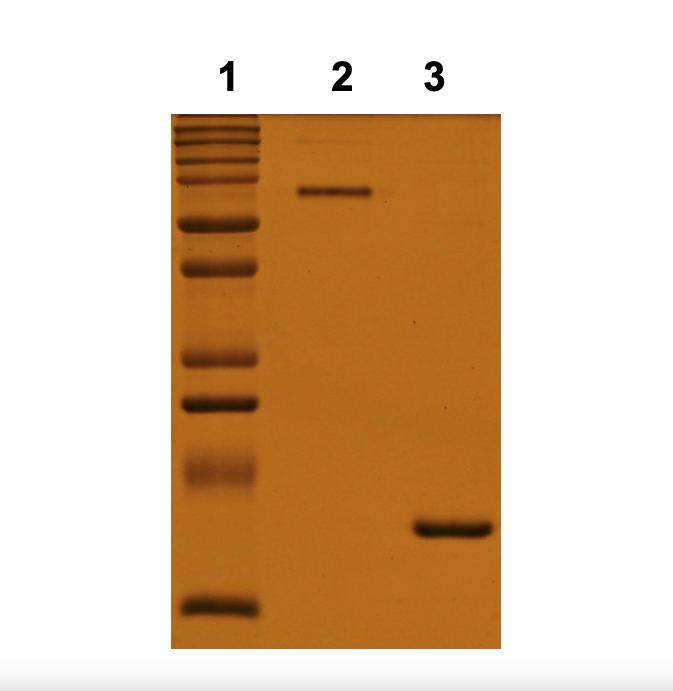
MicroProtein’s recombinant Streptavidin lacks carbohydrate side chains or other associated cofactors. Streptavidin is a 52 K Dalton, is a tetramer complex composed of 4 identical polypeptide chains. Recombinant Streptavidin protein contain no cysteine residues, sugar side chains or other cofactors. Streptavidin protein is stable over wide pH range and heat stable, heating up to 100°C for 20 minutes in 0.2% SDS to dissociate the subunits.
Excellent Functional Activity and Stability Our recombinant streptavidin protein is engineered for excellent performance. Whether you’re conducting research, diagnostics, or bioprocessing, count on MPT’s streptavidin to deliver consistent results.

- Robust Binding: Streptavidin’s strong affinity for biotin makes it an essential tool in various applications.
- Versatile Applications: Use it in ELISA, Western blotting, immunohistochemistry, Medical diagnostics, delivery drugs and more.
- Stable Performance: Our protein maintains its activity even under challenging conditions.
- Quality Assurance: Rigorous quality control ensures reliability and reproducibility.
- Nanotechnology: Use it in the design of biosensors, nanoparticles, and other nanoscale devices due to its strong biotin-binding capability.
- Proteomics: MPT’s streptavidin is used in proteomics studies to capture and purify biotinylated proteins and nucleic acids. It enables efficient enrichment of target proteins from complex mixtures.
- Mass sensitive application: MPT’s Molecular Grade Streptavidin Protein sets the standard for purity, stability, and is among the cleanest in industry. It is free from nuclease contaminants, ensuring top-tier performance in various applications. The protein is highly soluble in salt-containing buffers or water, achieving concentrations of up to 50 mg/ml or more. For custom solutions, MPT can provide streptavidin protein in buffer solutions up to 75 mg/ml, tailored to your specific needs.
Applications
MicroProtein’s high quality recombinant Streptavidin is a suitable tool for allowing universal test systems in molecular diagnostics, reagents, kits, and immunology. This recombinant Streptavidin is appropriate for coating solid phases such as microarrays, beads, and microplates etc.
Animal component free (ACF) & Animal origin free (AOF)
Read more about applications for streptavidin
Activity
Specific activity 17U/mg
Formulation
Lyophilized with 10% NaCl. For every 10 mg of lyophilizate, 10 mg consists of Streptavidin, with an additional 1 mg of NaCl.
Purity
≥95% Purity by SDS Page
Storage
Store at 4°C to -20°C. After reconstitution use within 2 weeks, store at 4°C.
50mg Package
Recombinant Streptavidin
Formulation: Lyophilized w/ NaCl
Product#: MPT-SA-50
$375.00 USD each
100mg Package
Recombinant Streptavidin
Formulation: Lyophilized w/ NaCl
Product#: MPT-SA-100
$450.00 USD each
1gram Package
Recombinant Streptavidin
Formulation: Lyophilized w/ NaCl
Product#: MPT-SA-1
$2340.00 USD each
Bulk Size
Recombinant Streptavidin
Formulation: Lyophilized w/ NaCl